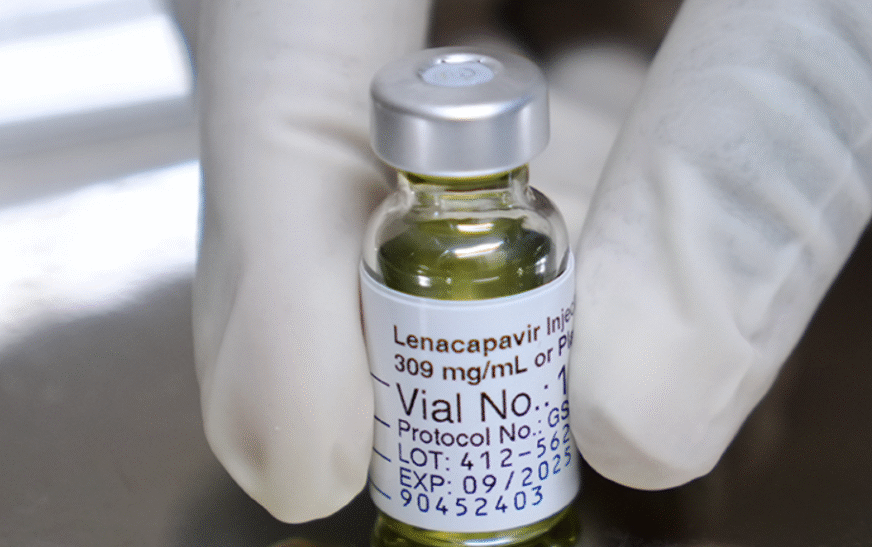New six months HIV Injectables cost Shs 100 million
The United States Food and Drug Administration (FDA) has approved long-acting injectable lenacapavir for HIV prevention. The new medicine is administered by injection once every six months and is a significant step in improving prevention options for people at risk of HIV infection around the world. In an interview, Gilead
US FDA Accepts Uganda’s Dei BioPharma’s Application for Diabetes and Weight Loss Drug
The U.S. Food and Drug Administration (FDA) has accepted an Abbreviated New Drug Application (ANDA) from Uganda’s Dei BioPharma for liraglutide, a multibillion-dollar treatment for diabetes and weight loss, marketed under the brand name Victoza. The FDA’s decision, officially recorded on April 23, 2025, clears a path for Dei BioPharma