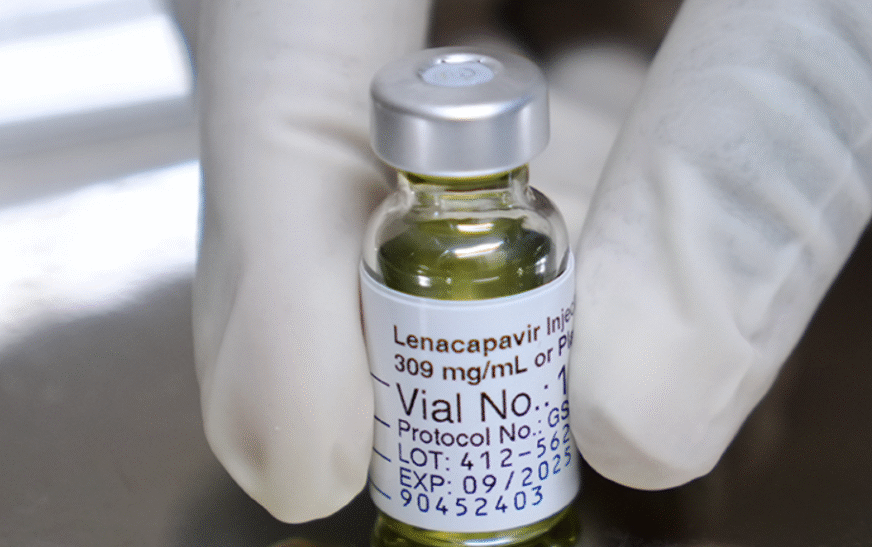Gov’t Approves Use of HIV injectables in Uganda
Uganda’s National Drug Authority (NDA) has approved the use of lenacapavir, a twice-yearly HIV prevention (PrEP) injection manufactured by Gilead Sciences, a U.S.-based company. The approval is a game-changer for HIV prevention, especially for people at high risk of infection. It marks a significant step toward ending AIDS by 2030.
New six months HIV Injectables cost Shs 100 million
The United States Food and Drug Administration (FDA) has approved long-acting injectable lenacapavir for HIV prevention. The new medicine is administered by injection once every six months and is a significant step in improving prevention options for people at risk of HIV infection around the world. In an interview, Gilead