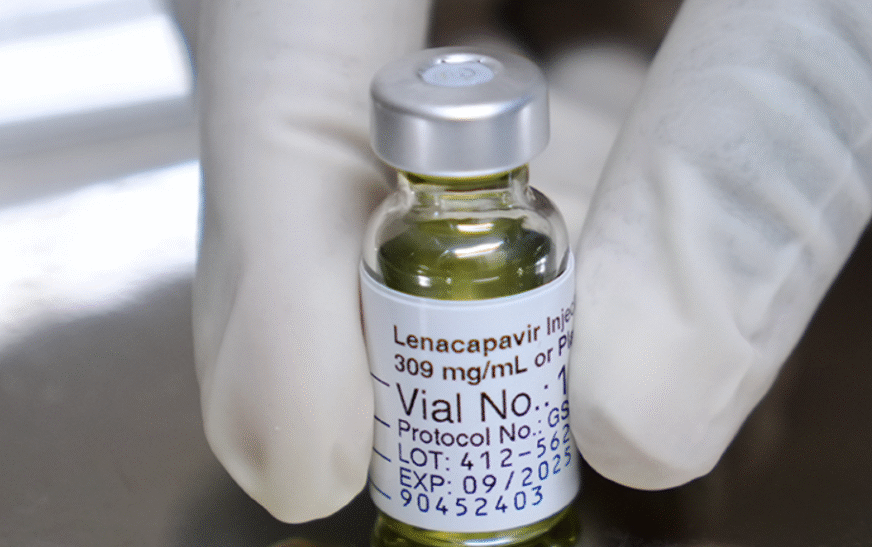The United States Food and Drug Administration (FDA) has approved long-acting injectable lenacapavir for HIV prevention.
The new medicine is administered by injection once every six months and is a significant step in improving prevention options for people at risk of HIV infection around the world.
In an interview, Gilead Sciences has announced a US list price of $28,218 (101,574,641) per person per year.
In a research paper published in The Lancet HIV this week, experts found that generic lenacapavir could cost $35 (Shs 12,598)-$46 (Shs 165,583) per person-year. This could fall to $25 (Shs 89,991) per person-year for a committed demand of five to ten million people within the first year, bringing pricing in line with or lower than current oral PrEP.
Responding to news of Lenacapavir’s FDA approval, Winnie Byanyima, Executive Director of UNAIDS and United Nations Undersecretary-General, said:“This is a breakthrough moment. The approval of lenacapavir is a testament to decades of public investment, scientific excellence, and the contributions of trial participants and communities.”
“I congratulate Gilead and US partners for advancing this important innovation. Lenacapavir could be the tool we need to bring new infections under control – but only if it is priced affordably and made available to everyone who could benefit,” she said.
“UNAIDS has seen research that lenacapavir can be produced for just $40 per person per year, falling to $25 within a year of roll out. It is beyond comprehension how Gilead can justify a price of $28,218. If this game-changing medicine remains unaffordable, it will change nothing. I urge Gilead to do the right thing. Drop the price, expand production, and ensure the world has a shot at ending AIDS.”